On December 17, Almazov Centre hosted the symposium Single-Cell Profiling in Oncology and the Novartis interactive symposium Fundamentals of Organizing CAR-T Therapy in a Clinical Centre. Both events were held as part of the programme of the World-Class Research Centre for Personalized Medicine.
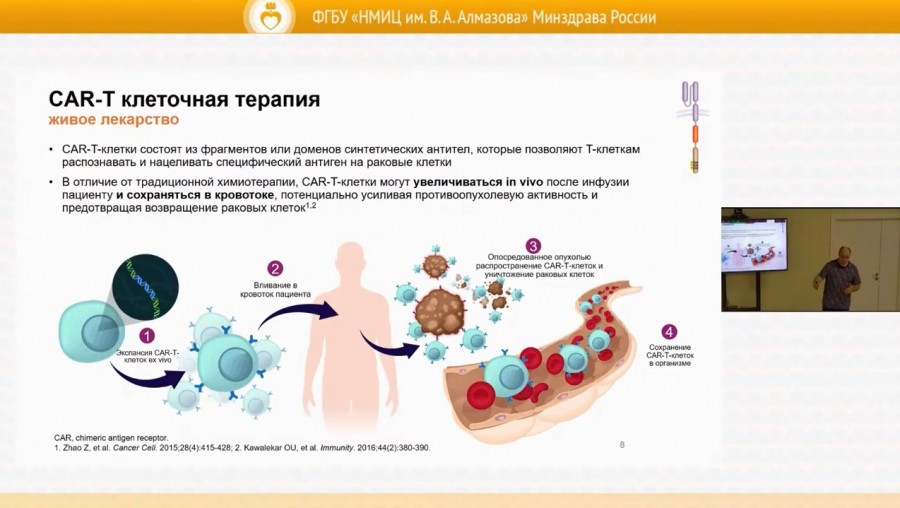
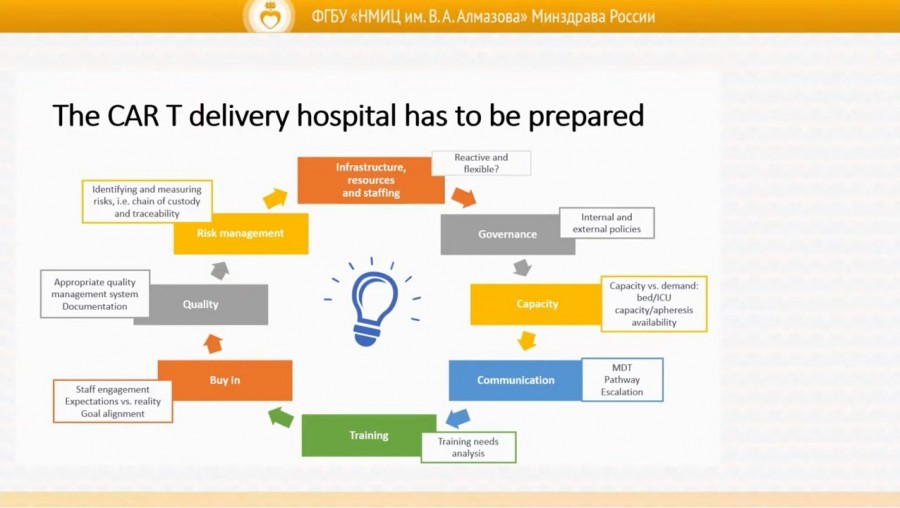
The industrial production of a CAR-T cell drug product is a staged process that includes clinical development, logistics and manufacturing. A well-structured process of interaction between departments and specialists of a clinical centre with each other and with other institutions (referring a patient for CAR-T cell therapy), skilled production of apheresis products and proper management of adverse events of CAR-T therapy are the key to the success of this innovative treatment.
The participants of the satellite symposium spoke about the features of CAR-T studies in Russian institutions. Expert from the UK Dr. Wendy Osborne shared her experience of organizing a multidisciplinary CAR-T team in her clinic (Freeman Hospital, Newcastle) and discussed with Almazov Centre specialists the peculiarities of working with CAR-T cells in a multidisciplinary facility.
Live broadcast:
General partner