Specialists of Polenov Neurosurgical Institute started a clinical trial for the «Development of a neurostimulation device for the regulation of motor functions in patients with the consequences of acute cerebrovascular accident.»
According to the World Health Organization, stroke remains the second leading cause of death worldwide. About half of stroke survivors suffer from severe motor impairments (paralysis and paresis). Despite the existing rehabilitation programs in the early post-stroke period (up to 6 months), many patients still have movement disorders leading to a worse quality of life. Movement disorders increase the risk of falling, lead to fear of loss of balance, additional disability and, as a result, to social isolation. The development of new, effective and safe technologies for the restoration of motor functions is a priority task of modern medicine.
The trial aims to test the effectiveness and safety of a non-invasive method of multisegment transcutaneous electrical spinal cord stimulation (tESCS) using a neuroprosthesis in patients with hemiparesis to restore walking ability and movement in a paretic (paralyzed) hand.
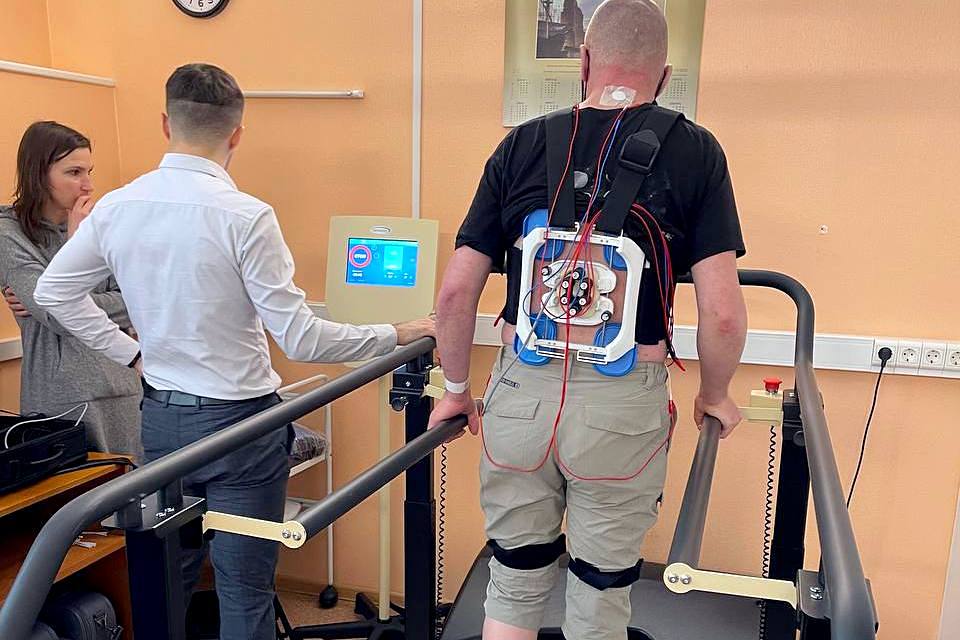
The operating principle of the neuroprosthesis is based on spinal cord stimulation through the skin, without implantation. Such stimulation leads to the activation of innate interlimb motor synergies. The neuroprosthesis is designed to not only improve the coordination, stability and speed of independent walking of patients, but also increase the motor function of the hand.
The clinical trial uses a system that allows simultaneous recording of kinematic indicators and EMG during walking. Biometric sensors are used to record walking parameters in the system. These miniature devices are mounted on the legs, hips and back of the patient and record the speed of rotation and acceleration in three axes, as well as EMG in two channels. Recordings using this system are combined with the main functional tests (10-meter and 6-minute walk tests).
Neuroprosthesis is a non-invasive device (short-term use of up to 1 hour) that is placed on intact skin and does not have direct contact with vital organs, systems and body cavities. Each patient is tested to determine whether an allergic reaction may develop at the site of electrode attachment. Cases of an allergic reaction to the electrodes and gel are rare, but if it occurs, the stimulation procedure is stopped.
Each day of the trial, the patient is accompanied by a methodologist or investigator who correct the patient's walking if necessary and ensure safety (physical control and verbal prompts in case of loss of balance).
“It is planned that about 20 patients will take part in the trial. If the spinal neuroprosthesis proves to be an effective method of recovery, we will consider including it in the list of high-tech medical devices,” explains Head of the Medical Rehabilitation Department of Polenov Neurosurgical Institute, Dr. E.N. Zharova.

